lewis dot structure for h2s|Lewis Structure of H2S (With 6 Simple Steps to Draw!) : Pilipinas Your final Lewis structure for H2S should look like this: FAQs. 1. What is the Lewis structure of H2S? The Lewis structure of H2S represents the arrangement of atoms . Watch Napa.iyak sa sakit sa sobrang laki ng titi on Kantotin. You can also watch hundreds of pinay sex scandals, premium porn and amateur sex videos in this site.
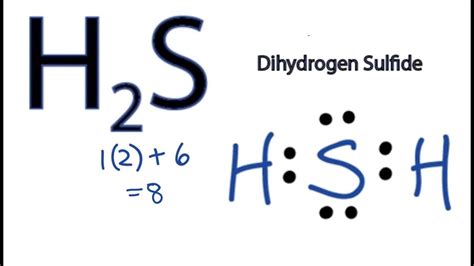
lewis dot structure for h2s,A step-by-step explanation of how to write the Lewis Dot Structure for H2S (Dihydrogen Sulfide). The H2S Lewis structure is similar to the structure for water H2O since Sulfur (S) and (O) are.Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is .
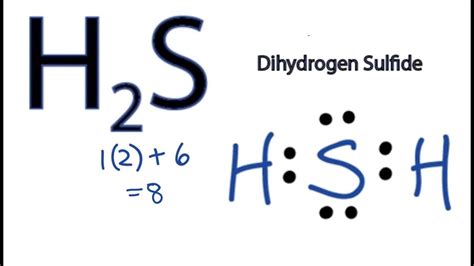
Your final Lewis structure for H2S should look like this: FAQs. 1. What is the Lewis structure of H2S? The Lewis structure of H2S represents the arrangement of atoms . Lewis structure of H2S contains single bonds between the Sulfur (S) atom and each Hydrogen (H) atom. The Sulfur atom (S) is at the center and it is . What is the Lewis structure of hydrogen sulfide H2S? How to Draw the Dot Structure for H2S; Step 1: Count the total number of valence electrons; Step 2: . The Lewis Structure of Hydrogen Sulfide is easy to draw and understand. In this compound, both the hydrogen atoms require one electron to make the covalent bond with Sulfur. The Lewis structure of .
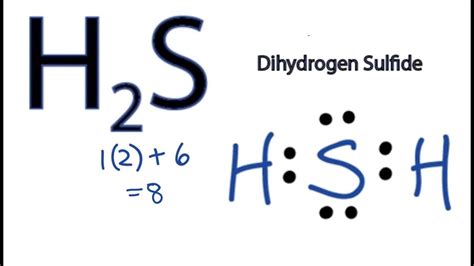
In the H 2 S Lewis structure, there are two single bonds around the sulfur atom, with two hydrogen atoms attached to it, and on the sulfur atom, there are two lone .Let's do the Lewis structure for H2S: Dihydrogen Sulfide. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that .
lewis dot structure for h2s How do you arrange the electrons around the atoms of Hydrogen sulphide? Watch this video to learn how to draw the Lewis dot structure of H2S, a simple but .
Let's do the Lewis structure for H2S: Dihydrogen Sulfide. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Total of 8 valence electrons. Let's draw this thing. We'll put Sulfur here. The Lewis structure of hydrogen sulfide is best represented as a bent H{eq}_2 {/eq}S molecule with two lone pairs of electrons on the S atom represented by two pairs of dots (or two bars). . Step 3: Connect the atoms. Draw the central sulfur atom and the two hydrogen atoms around it. Connect each hydrogen atom to the sulfur atom with a single bond. This will use up two valence electrons, leaving six more. Lewis structure of hydrogen sulfide H 2 S (step 3 draw single bonds) Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. This page titled 9.2: Lewis Electron Dot Diagrams is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, . The Lewis dot structure of hydrogen sulfide (H 2 S) consists of a sulfur (S) atom at the center. It is surrounded by 2 hydrogen (H) atoms, one on each side, by a single covalent bond. The central S-atom carries 2 lone pairs of electrons, while there is no lone pair on any of the terminal H-atoms.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. . Chemists normally represent a bond using a line instead of two dots. The structures of H 2, F 2, and H 2 O would usually be drawn as follows: Only the bonding electrons are .
Molecular Geometry of Hydrogen Sulfide (H. S) Hydrogen sulfide (H 2 S) molecule consists of one sulfur (S) atom and two hydrogen (H) atoms. Hydrogen (H) is located in Group 1, and sulfur (S) is in Group 16 of the periodic table. Hydrogen has one, and sulfur has six valence electrons. The total number of valence electrons in hydrogen sulfide is 8.
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with .The Lewis Dot Structure for H 2 S:. Hydrogen sulfide (H 2 S) has the distinctive odor of rotten egg gas, and is produced by some organisms during metabolism, as well as being emitted by volcanic gasses. The Lewis structure shows how the valence electrons are used to make two chemical bonds. Answer and Explanation: 1In the H2S Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the H2S Lewis structure, with all two hydrogen atoms arranged in a tetrahedral geometry. Add valence electron around the hydrogen atom, as given in the figure. Step-3: Lewis dot Structure for H2S generated from step-1 and .
This is the Lewis Dot Structure for H2S (hydrogen sulfide). The rules for drawing lewis structures permit the replacement of the bond lines with two electrons. As we have previously discussed, hydrogen . In the H2S lewis structure, the intermixing 3s, 3p orbital form sp3 hybridized orbital, so the covalent bond angle should be 109.5̊ but it is lowered to 92.1̊ by the steric repulsion between dense two non bonding electron pair of ‘S’. . the angle between the two nonbonding electron pairs increases for stabilizing the electron dot .Draw the electron dot stucture of. H. 2. S. (hydrogen sulphide)? Solution. Verified by Toppr.
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .
Lewis structure of B e F 2: State whether true or false : View Solution. Q4. Draw the Lewis structure for C O 2 . H2S lewis structure has a Sulfur atom (S) at the center which is surrounded by two Hydrogen atoms (H). There are 2 single bonds between the Sulfur atom (S) . In the above lewis dot structure of H2S, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following lewis .Lewis Structure of H2S (With 6 Simple Steps to Draw!) A Lewis dot structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. . Draw Lewis dot structures for two hydrogen atoms and one oxygen atom. Attempt to arrange these three atoms so that they are sharing electrons. A .
Interpreting Lewis Structures. A Lewis structure contains symbols for the elements in a molecule, connected by lines and surrounded by pairs of dots. For example, here is the Lewis structure for water, H 2 O. Each symbol represents the nucleus and the core electrons of the atom. Here, each “H” represents the nucleus of a hydrogen atom, .
lewis dot structure for h2s|Lewis Structure of H2S (With 6 Simple Steps to Draw!)
PH0 · Lewis structure of hydrogen sulfide H2S
PH1 · Lewis Structure of H2S (With 6 Simple Steps to Draw!)
PH2 · Hydrogen Sulfide (H2S) Lewis Structure
PH3 · H2S Lewis structure
PH4 · H2S Lewis Structure: How to Draw the Dot Structure for H2S
PH5 · H2S Lewis Structure, Molecular Geometry,
PH6 · H2S Lewis Structure, Molecular Geometry
PH7 · H2S Lewis Structure
PH8 · H2S (Hydrogen sulfide) Lewis Structure
PH9 · Draw the Lewis dot structure of Hydrogen sulphide molecule